Preserving the Black-Footed Ferret Species Through Gene Cloning and Genetic Engineering
By Melanie Riley
History of the Black-Footed Ferret
Native to North America, black-footed ferrets have existed for about 30,000 years.1 Over the years, many changes have happened, most coming from the hands of humans.

Prairie Dog
Photograph courtesy of Mark Gocke
Black-footed ferrets consume prairie dogs, so the population of prairie dogs and black-footed ferrets correlate. As ranchers and farmers began settling the land, they started poisoning prairie dogs to reduce the population size because they were considered pests. Today, prairie dogs are still sometimes considered pests, but there has been ongoing research on the importance of prairie dogs to the ecosystem, such as food for black-footed ferrets and providing homes or dens for animals including the ferret, burrowing owls, and snakes. With the decrease of prairie dogs, the population of black-footed ferrets began to decline. In the 1970s, the black-footed ferret species was thought to be extinct until 1981, when a dog changed everything.
Rediscovery
On the Hogg’s Ranch in Meeteetse, Shep, the cattle-dog, was playing with an animal late one night in 1981. The next morning, Shep’s owners found what he was playing with. It was an animal they had never seen before. After asking Game and Fish and others in town, the final conclusion was that it was a black-footed ferret. This species was just about to be claimed as extinct. Wildlife researchers arrived in the small town of Meeteetse, waited till the dark of night fell and went out to the ranch to look for the ferrets with flashlights. At night and in the light of a flashlight, their green eyes can be observed due to “a reflective layer in the back of the eye called the tapetum lucidum.”2 This is latin for “bright tapestry” and is beneficial for night vision. The researchers quickly saw these green eyes, along with a black mask, black feet and black tail tip. The ferret ran by and into a hole. One of the researchers ran to the hole to see if he could see it; to his surprise, the ferret came out and barked at him. At that moment, they knew that black-footed ferrets were not extinct and ideas started rolling on finding how many were actually alive.

Capturing the Black-Footed Ferrets
Photograph courtesy of Mark Gocke
A few years after rediscovery, the population of black-footed ferrets in Meeteetse began to decline even more. This decline came from canine distemper and the sylvatic plague. The sylvatic plague is “a flea-borne bacterial disease of wild rodents. Prairie dogs and black-footed ferrets are susceptible to the disease”.3 Prairie dogs would contract the disease from fleas then black-footed ferrets would get sick from eating the prairie dogs. To preserve the species of black-footed ferrets, humans intervened and set-up a captive-breeding program. They caught the remaining eighteen ferrets in Meeteetse and took them in. The program has been a huge success. In the program, ferrets were given vaccinations for canine distemper and the sylvatic plague. After years of breeding and growing the population, they were reintroduced into Meeteetse in 2016.

Ferret in Captive Breeding
Photograph courtesy of Mark Gocke
Today, the black-footed ferret population in Meeteetse is still thriving and growing; however, upon further research and studies, the species of BFFs might not last long. There are two main things that are a threat to this species: 1) the lack of genetic diversity and 2) the sylvatic plague.
Genetics Introduction
Before going on any further, it is important to have some information about chromosomes and genetics. DNA contains the genetic information of animals and humans. It is unique for each person and animal. DNA gets compacted into chromosomes and is found inside the nucleus of a cell. DNA is sectioned into genes on the chromosome. Genes code for a different trait i.e. eye color, hand size, cell structure, etc. Different forms or varieties of the same gene are called alleles. For example, with eye color, there are different color varieties: blue, green, brown, etc. Therefore, genes are specific traits while alleles are the specific forms the gene takes. Genetic information gets passed from mother and father to offspring through genes on the chromosomes. Information that gets passed down are things like eye color, height, and health. With each allele that gets passed on, they can either be the same from both parents or different. This is how alleles make someone unique.
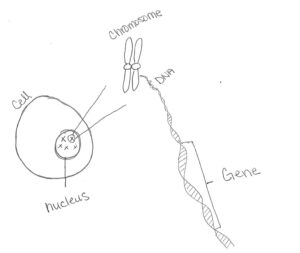
Image 1: DNA, Chromosome and Gene
Drawing by Melanie Riley
Reginald Crundall Punnett created the Punnett square which can be used to predict the variations of genetic information that can come from cross breeding. For the Punnett square, allele information is collected from the mom and dad. It can then be used to predict the genetic information their offspring could have. Alleles are separated into two classifications: dominant and recessive. Dominant alleles completely overrule recessive alleles. Therefore, these classifications are named depending on what is actually physically presented. Alleles come in pairs that can be either heterozygous (different alleles) or homozygous (same alleles). One allele comes from one parent and the other allele comes from the other parent. Heterozygous pairs have a dominant and a recessive allele. Homozygous pairs will either have two dominant alleles or two recessive alleles. The dominant trait will be present in heterozygous pairs. In homozygous dominant pairs (having two dominant alleles), the dominant allele will be present and in homozygous recessive alleles (having two recessive alleles), the recessive allele will be present. For use of the Punnett square, dominant alleles are labeled with a capital letter and recessive alleles are labeled with a lowercase letter.
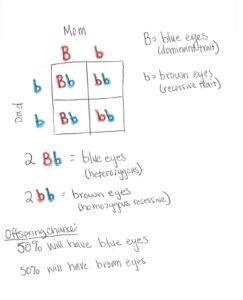
Using the Punnett square to predict offspring alleles.
Drawing by Melanie Riley
A popular example using the Punnett square is eye color. In this example, blue eyes are going to be dominant and labeled as (B) and brown eyes are going to be recessive and labeled with (b). The mom’s two alleles are Bb and the dad’s are bb. This means the mom’s alleles are heterozygous, but since B (blue eyes) are dominant, she has blue eyes. The dad’s alleles are homozygous recessive. Since they are both recessive, that means his eyes are brown (b). As shown in image 2, the mom’s alleles are on the top row and the dad’s are along the side. Each box can only have two letters in it. One letter is taken from mom and one from dad. This process is repeated for each box. Once that is completed, the predicted genetic information their offspring could have is 50% heterozygous like mom with blue eyes and 50% homozygous recessive like dad with brown eyes. That means that each child would have a 50% chance to be either heterozygous or homozygous.
Genetics of the Black-Footed Ferret
The passing of genetic information occurs in black-footed ferrets as well. Black-footed ferrets have 38 chromosomes making 19 pairs of chromosomes.4 This is different from humans who have 46 chromosomes (23 pairs). These pairs can either be heterozygous (different alleles) or homozygous (same alleles) as mentioned earlier in this article. The black-footed ferrets that are alive today in the United States come from a foundation of seven ferrets. Genetic testing has provided this information. Testing has also shown that the black-footed ferrets living today have genetic information that is very closely related to each other. There are signs that inbreeding is happening among the species. Inbreeding increases homozygosity in offspring. Homozygosity is having too many alleles that are the same. This presents problems because it leads to a lack of genetic variation and more genetic mutations. Genetic mutations occur when the DNA gets changed causing genes to not code for the right trait. There are consequences of inbreeding such as decreased health, fitness ability, and offspring production. Thus, if inbreeding continues, extinction may happen because they will not be able to recover from sickness, have the physique to protect themselves, or to produce more offspring.
Genetic Cloning Process
Genetic restoration and cloning has been researched and successfully conducted on other animals. Genetic restoration is when genes are restored back into the population. Genetic cloning is for genetic information to be duplicated from one animal to another. Somatic cell nuclear transfer (SCNT) uses somatic cells, like skin cells, instead of germ cells (sperm and eggs) for cloning. It is beneficial because sometimes germ cells are not able to be collected for certain reasons such as the animal cannot reproduce, the opportunity to collect them is not available anymore, or they were not collected while the animal was alive. This process involves taking a donor oocyte (a female egg) from the same species, removing the nucleus and adding somatic cells from collected culture to the oocyte. After the addition, these oocytes develop into reconstructed embryos and are cultured into blastocysts in vitro and are then placed in a surrogate female through surgery. This process does not always produce a living clone – difficulties and problems can arise that cause embryos not to develop. But there is value in gene cloning, especially for black-footed ferrets. It is species-specific, meaning that with each species different techniques will be used and differing results are produced.
Interspecies somatic cell nuclear transfer (iSCNT) was used for the cloning process of black-footed ferrets. This is very similar to SCNT, however, it involved taking an oocyte from a closely-related, non endangered species. (Image 3). The oocyte was then placed in the closely-related, non-endangered surrogate mother. It goes through the same steps and process as SCNT. The need for iSCNT was to not harm an already endangered species.
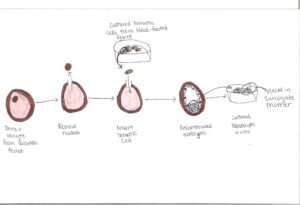
Image 3: iSCNT process
Drawing by Melanie Riley
To begin iSCNT in black-footed ferrets, the choice of donor oocyte and surrogate needed to be made. The steppe polecats are the closest related species to the black-footed ferrets. The next closely related are the domestic ferrets. Domestic ferrets were chosen as the surrogate because they were already being used in reproductive cloning, therefore more knowledge and technical aspects had already been gathered. Polecats were not used because problems arose when testing them. Black-footed ferrets were not used either because surgery presented a risk of death to an already endangered species. Before beginning this process, there needed to be an understanding of problems like improper genetic programming that could happen as well as ways to limit problems that may arise. “Genetic reprogramming is a critical step in early embryonic development that relies on epigenetic interactions of maternal cytoplasmic RNA and proteins to remove and remodel the existing methylation pattern on the inherited, or in the case of reproductive cloning, injected chromatin… Recent research has shown that inappropriate genetic reprogramming can be mitigated with chemical treatment of donor chromatin to change methylation patterns involved in gene expression”.5 Another problem to be overcome is the natural process of the black-footed ferret nuclear DNA mixing with the mitochondrial DNA from the donor species. To produce a black-footed ferret with native cytoplasmic features, a plan of breeding and mating would need to take place. After the birth of cloned black-footed ferrets, they would need to be observed for abnormalities during their lifetime. If there were no abnormalities, then steps could be taken to introduce the genes into the captive breeding population and reintroduce them to the wild. This would be the ultimate way that the black-footed ferret species could survive.
The Birth of Elizabeth Ann

Ben Novak, the lead scientist of Revive & Restore, excitedly meeting Elizabeth Ann on December 31st, 2020. Little Elizabeth Ann was just 21 days old.
Courtesy of Revive & Restore
Tissue samples of Willa, a black-footed ferret that was captured from Meeteetse, were cryopreserved at the time she passed away in 1988. Willa’s genome contained “three times more unique variations than the living population”.6 To cryopreserve the tissue sample, they had to be culturized. Culturalization of somatic cells involves collecting a small tissue sample through biopsy, cutting the pieces into 1 mm^2 pieces, dispensing the cell by digesting it with an enzyme, placing it in flasks with media where the cells will attach and divide, then cryopreserving in a freezer.7
In 2020, these frozen cryopreserved cells were thawed with cryoprotectants and used to produce a cloned black-footed ferret. A baby black-footed ferret was born December 10, 2020 through a domestic ferret surrogate mother. This baby was named Elizabeth Ann and is the exact clone of Willa. Elizabeth Ann has been observed through her lifetime and there have not been any abnormalities. They placed her with a few mates for breeding purposes, however, she needed an emergency ovariohysterectomy and cannot be bred. She is still alive today and in Colorado at a conservation center.
Are There Others?
The success that has come from Elizabeth Ann shows that genetic cloning and iSCNT can be done in this species.
There are genetic twins of Elizabeth Ann that can be used for genetic cloning and iSCNT. There is a male from the original capture in 1981 that can be used as well. He has different genetics than Willa and the living ferrets which can further aid genetic diversity. It will take many years of experimenting, breeding, and observing before any cloned black-footed ferret can be placed in the wild. But there is hope for the survival of this species. If everything does go well with Willa and the male’s genetics, then there will be nine founders instead of seven providing more genetic diversity.
There is a negative side to using the male’s genetic information, however. A few days after receiving his biopsy, he passed away from canine distemper. Canine distemper is a highly contagious viral disease. There may be the chance that his cells could be infected. There are possible ways to go about removing the infection if it is present in the cells. Before any cell does get used from him, it will need to go through testing to see if it does have the infection.
Genetic Engineering
As mentioned earlier, sylvatic plague was a cause of the decline of the black-footed ferret population in the 1980s and is still a problem today. Scientists have developed a vaccine for the species to protect them against the plague. For future survival, humans will always need to have a hand in preserving this species by giving vaccinations. That is unless something else can be done about it.
Genetic engineering is being looked into as a solution to limit human interaction with black-footed ferrets. Genetic engineering is changing the genetic DNA makeup of an organism. This could be used by altering the gene response to sylvatic plague and creating immunity in the black-footed ferret species. Once genes pass on, then the population will become immune and not be affected by the plague. This does have consequences and further testing and research will need to happen before changing the genetic makeup of black-footed ferrets.
Conclusion
In conclusion, black-footed ferrets have been around for many years and are endangered now due to issues like lack of prairie dogs for food, canine distemper, the sylvatic plague, and lack of genetic diversity. There have been positive movements to increase the population and there are many more things to be done to preserve this endangered species. These options are continued genetic cloning and iSCNT as well as genetic engineering and vaccinations. Through science and wildlife management, black-footed ferrets can thrive in the wild again like they used to.

A Black-Footed Ferret looking at camera
Photograph courtesy of Mark Gocke
Bibliography
- McClure, Nancy. “The Black-Footed Ferret: Here, Gone, and Back Again.” Buffalo Bill Center of the West, 18 Jan. 2021, centerofthewest.org/2017/12/01/points-west-black-footed-ferret/.
- Hartogh, Frances. “Curious Nature: Are Those Glowing Eyes a Bear or Mountain Lion? Eye Shine Color Could Crack the Code.” SummitDaily.Com, 1 Nov. 2022, www.summitdaily.com/opinion/curious-nature-are-those-glowing-eyes-a-bear-or-mountain-lion-eye-shine-color-could-crack-the-code/#:~:text=Eyeshine%20is%20caused%20by%20a,of%20tissue%20behind%20the%20retina.
- “Sylvatic Plague.” Sylvatic Plague | U.S. Geological Survey, www.usgs.gov/programs/fish-and-wildlife-disease/sylvatic-plague. Accessed 17 May 2023.
- Nona. “Black-Footed Ferret (Mustela Nigripes).” All About Ferrets, 6 Sept. 2017, all-about-ferrets.com/black-footed-ferret.html
- Wisely, Samantha M., et al. “A Road Map for 21st Century Genetic Restoration: Gene Pool Enrichment of the Black-Footed Ferret.” Journal of Heredity, vol. 106, no. 5, 24 Aug. 2015, pp. 581–592, https://doi.org/10.1093/jhered/esv041.
- “Genetic Research Boosts Black-Footed Ferret Conservation Efforts: U.S. Fish & Wildlife Service.” FWS.Gov, 18 Feb. 2021, www.fws.gov/press-release/2021-02/genetic-research-boosts-black-footed-ferret-conservation-efforts.
- “From the Big Picture to the Itty-Bitty Picture in Black-Footed Ferret Conservation: Discussion Panel.” YouTube, YouTube, 29 Sept. 2022, https://www.youtube.com/watch?v=pbY_HWLoTlE. Accessed 18 May 2023.
References
Biello, David. “De-Extinction in Action: Scientists Consider a Plan to Reinject Long-Gone DNA into the Black-Footed Ferret Population.” Scientific American, 1 Aug. 2016, www.scientificamerican.com/article/de-extinction-in-action-scientists-consider-a-plan-to-reinject-long-gone-dna-into-the-black-footed-ferret-population/.
“The Black-Footed Ferret Project.” Revive & Restore, reviverestore.org/projects/black-footed-ferret/. Accessed 25 May 2023.
Bron, Gebbiena M., et al. “Impact of Sylvatic Plague Vaccine on Non-Target Small Rodents in Grassland Ecosystems.” EcoHealth, vol. 15, no. 3, 2018, pp. 555–565, https://doi.org/10.1007/s10393-018-1334-5.
“Cloning.” MedlinePlus, medlineplus.gov/cloning.html#:~:text=Cloning%20describes%20the%20processes%20used,referred%20to%20as%20a%20clone. Accessed 17 May 2023.
“From the Big Picture to the Itty-Bitty Picture in Black-Footed Ferret Conservation: Discussion Panel.” YouTube, YouTube, 29 Sept. 2022, https://www.youtube.com/watch?v=pbY_HWLoTlE. Accessed 18 May 2023.
“Genetic Research Boosts Black-Footed Ferret Conservation Efforts: U.S. Fish & Wildlife Service.” FWS.Gov, 18 Feb. 2021, www.fws.gov/press-release/2021-02/genetic-research-boosts-black-footed-ferret-conservation-efforts.
Hartogh, Frances. “Curious Nature: Are Those Glowing Eyes a Bear or Mountain Lion? Eye Shine Color Could Crack the Code.” SummitDaily.Com, 1 Nov. 2022, www.summitdaily.com/opinion/curious-nature-are-those-glowing-eyes-a-bear-or-mountain-lion-eye-shine-color-could-crack-the-code/#:~:text=Eyeshine%20is%20caused%20by%20a,of%20tissue%20behind%20the%20retina.
“Importance of the Endangered Species Act.” Endangered Species Coalition, www.endangered.org/importance-of-the-endangered-species-act/#:~:text=Healthy%20ecosystems%20depend%20on%20plant,ecosystems%20to%20purify%20our%20environment. Accessed 17 May 2023.
Matchett, Marc R., et al. “Oral Sylvatic Plague Vaccine Does Not Adequately Protect Prairie Dogs (Cynomys Spp.) for Endangered Black-Footed Ferret (Mustela Nigripes) Conservation.” Vector-Borne and Zoonotic Diseases, vol. 21, no. 12, 2021, pp. 921–940, https://doi.org/10.1089/vbz.2021.0049.
McClure, Nancy. “The Black-Footed Ferret: Here, Gone, and Back Again.” Buffalo Bill Center of the West, 18 Jan. 2021, centerofthewest.org/2017/12/01/points-west-black-footed-ferret/.
Nona. “Black-Footed Ferret (Mustela Nigripes).” All About Ferrets, 6 Sept. 2017, all-about-ferrets.com/black-footed-ferret.html.
“Reginald Crundall Punnett.” Reginald Crundall Punnett :: DNA from the Beginning, www.dnaftb.org/5/bio.html#:~:text=Punnett%20devised%20the%20%22Punnett%20Square,the%20attention%20of%20English%20scientists. Accessed 17 May 2023.
Richgels, Katherine L., et al. “Evaluation of Yersinia Pestis Transmission Pathways for Sylvatic Plague in Prairie Dog Populations in the Western U.S.” EcoHealth, vol. 13, no. 2, 2016, pp. 415–427, https://doi.org/10.1007/s10393-016-1133-9.
Smith, Mike. “Genetic Engineering.” Genome.Gov, www.genome.gov/genetics-glossary/Genetic-Engineering#:~:text=Genetic%20engineering%20. Accessed 17 May 2023.
“Sylvatic Plague.” Sylvatic Plague | U.S. Geological Survey, www.usgs.gov/programs/fish-and-wildlife-disease/sylvatic-plague. Accessed 17 May 2023.
Szuszwalak, Joe. “Genetic Research Boosts Black-Footed Ferret Conservation Efforts: U.S. Fish & Wildlife Service.” FWS.Gov, 18 Feb. 2021, www.fws.gov/press-release/2021-02/genetic-research-boosts-black-footed-ferret-conservation-efforts.
Tripp, Daniel W., et al. “Burrow Dusting or Oral Vaccination Prevents Plague-Associated Prairie Dog Colony Collapse.” EcoHealth, vol. 14, no. 3, 2017, pp. 451–462, https://doi.org/10.1007/s10393-017-1236-y.
Wisely, Samantha M., et al. “A Road Map for 21st Century Genetic Restoration: Gene Pool Enrichment of the Black-Footed Ferret.” Journal of Heredity, vol. 106, no. 5, 24 Aug. 2015, pp. 581–592, https://doi.org/10.1093/jhered/esv041.
Photographs
Photographs courtesy of Mark Gocke of Wyoming Game and Fish Department:
- Prairie Dog
- Capturing the Black-Footed Ferrets
- Ferret in Captive Breeding
- A Black-Footed Ferret
Photograph of Ben Novak with Elizabeth Ann courtesy of Revive and Restore
Drawings by Melanie Riley

